



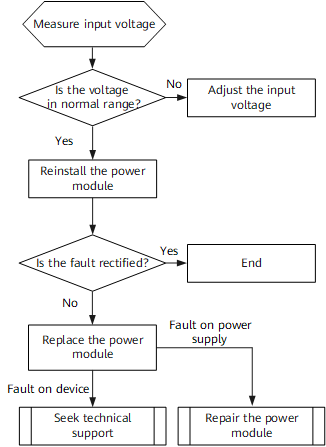

























The US Food and Drug Administration (FDA) published a guide for the public dealing with medical device cybersecurity. The guide contains three sections: (1) explanation about Internet connected medical devices and the related cybersecurity issues; (2) description of the FDA's role in regulating medical devices; (3) tips for the public on how to keep their devices safe. Among the tips provided to the public are the following: updating the devices' software, registering the device with the manufacturer, being aware if the device is not functioning appropriately, involving family or caregivers and educating them about the device, and in case of emergency to seeking medical assistance.
 Tags chauds:
Développement des capacités
La controverse.org
Protection des consommateurs
Internet des objets
Tags chauds:
Développement des capacités
La controverse.org
Protection des consommateurs
Internet des objets
Inscrivez-vous par courriel maintenant pour le Stock de Promotion hebdomadaire
100% free, Unsubscribe any time!
Add 1: Room 605 6/F FA YUEN Commercial Building, 75-77 FA YUEN Street, Mongkok KL, HongKong Add 2: Room 405, Building E, MeiDu Building, Gong Shu District, Hangzhou City, Zhejiang Province, China
Whatsapp/Tel: +8618057156223 Tél. : + 33 (0) 3 88 88 20: 0086 571 86729517 Tel à HK: 00852 66181601
Courriel:: [email protected]
 English
English Pусский
Pусский Français
Français Español
Español Português
Português